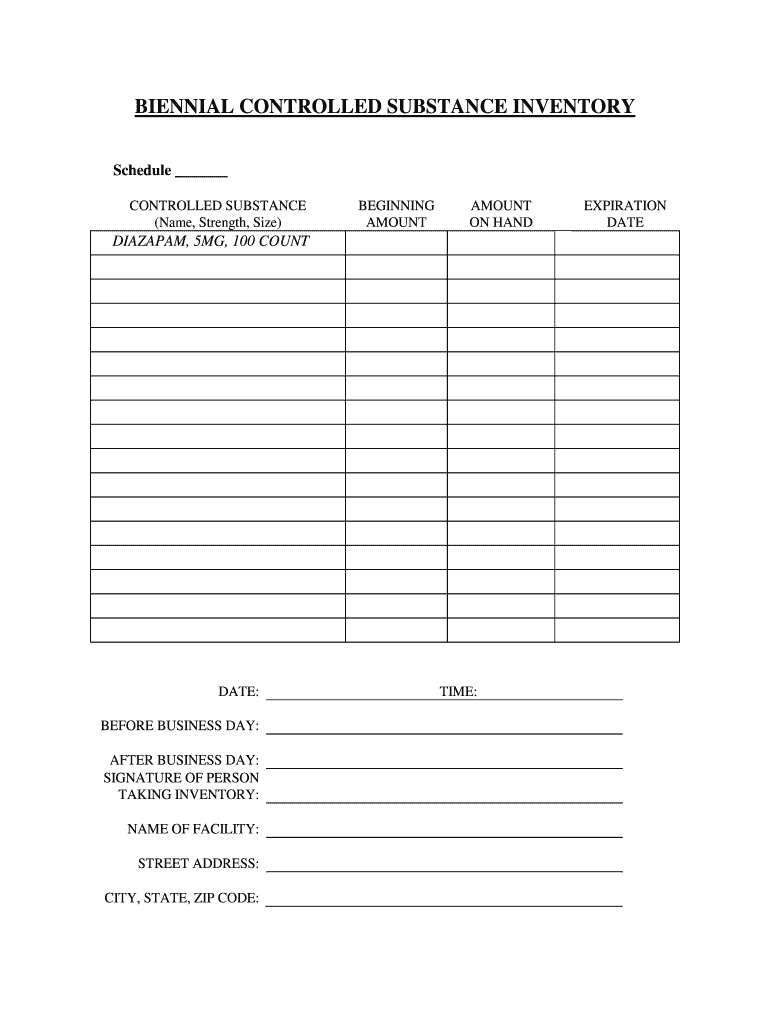
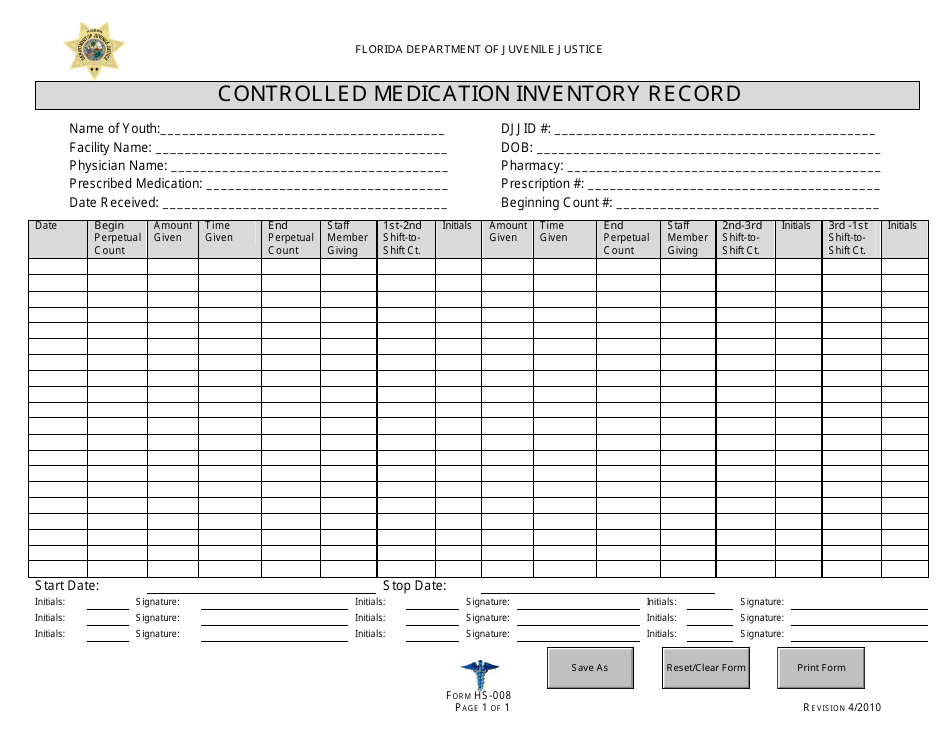
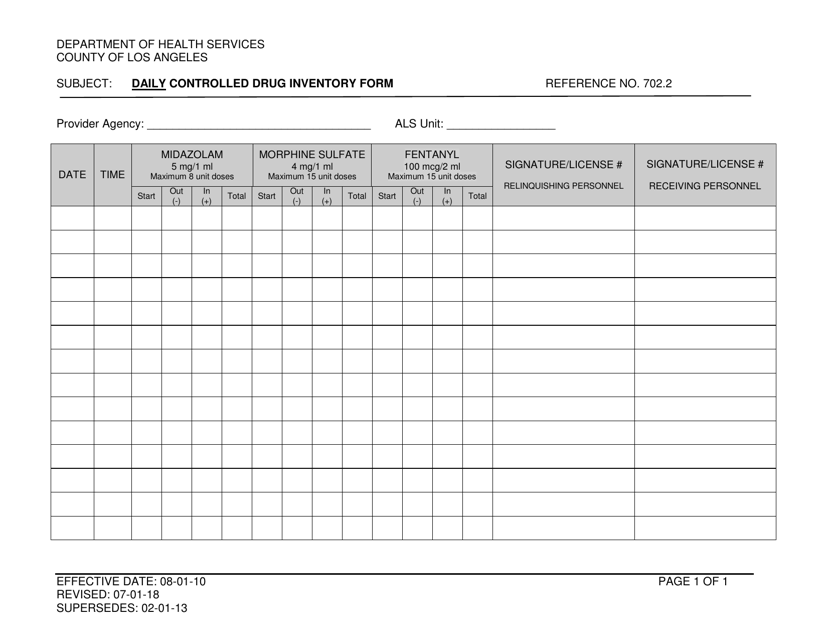
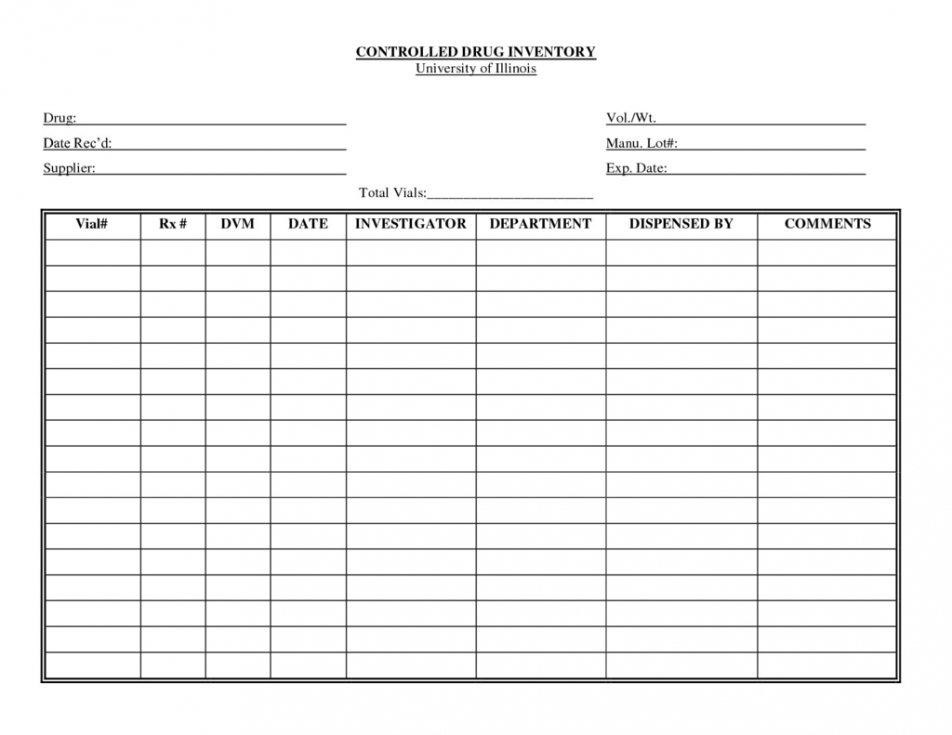
Controlled Substance Biennial Inventory Form
Controlled Substance Biennial Inventory Form - The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. The dea requires a physical inventory of all controlled substances to be conducted every. Biennial & initial controlled substance inventory form. See obp controlled substance inventory requirements. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. Controlled substances initial/biennial inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled.
Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. Biennial & initial controlled substance inventory form. The dea requires a physical inventory of all controlled. See obp controlled substance inventory requirements. Controlled substances initial/biennial inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each.
See obp controlled substance inventory requirements. Controlled substances initial/biennial inventory form. The dea requires a physical inventory of all controlled. This form can be used to complete initial, annual (obp), and biennial (dea) inventory. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Biennial & initial controlled substance inventory form. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from all other drugs or.
Biennial Inventory Form Fill Online, Printable, Fillable, Blank
Biennial & initial controlled substance inventory form. The dea requires a physical inventory of all controlled. (2) schedule i and ii drugs must be separated from all other drugs or. Controlled substances initial/biennial inventory form. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each.
Printable Controlled Substance Inventory Log
(2) schedule i and ii drugs must be separated from all other drugs or. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Dea biennial controlled.
Dea biennial inventory form Fill out & sign online DocHub
(2) schedule i and ii drugs must be separated from all other drugs or. Biennial & initial controlled substance inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Each inventory shall contain a.
Printable Controlled Substance Inventory Log
Controlled substances initial/biennial inventory form. The dea requires a physical inventory of all controlled. (2) schedule i and ii drugs must be separated from all other drugs or. This form can be used to complete initial, annual (obp), and biennial (dea) inventory. See obp controlled substance inventory requirements.
2014 Temple University Biennial Controlled Substance Inventory Form
The dea requires a physical inventory of all controlled. Biennial & initial controlled substance inventory form. See obp controlled substance inventory requirements. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. (2) schedule i and ii drugs must be separated from all other drugs or.
Printable Controlled Substance Inventory Log
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Biennial & initial controlled substance inventory form. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. The dea requires a physical inventory of all controlled. Controlled substances.
Free Printable Controlled Substance Log
The dea requires a physical inventory of all controlled. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. See obp controlled substance inventory requirements. This form.
Free Biennial Controlled Substance Inventory Form New 19 Of Controlled
The dea requires a physical inventory of all controlled substances to be conducted every. Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken, and. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i.
free biennial controlled substance inventory form new 19 of controlled
(2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Controlled substances initial/biennial inventory form. The dea requires a physical.
Fillable Online controlled substances initial/biennial inventory form
(2) schedule i and ii drugs must be separated from all other drugs or. Biennial & initial controlled substance inventory form. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. This form can be used to complete initial, annual (obp), and biennial (dea) inventory. See obp controlled.
See Obp Controlled Substance Inventory Requirements.
This form can be used to complete initial, annual (obp), and biennial (dea) inventory. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or. Biennial & initial controlled substance inventory form.
Each Inventory Shall Contain A Complete And Accurate Record Of All Controlled Substances On Hand On The Date The Inventory Is Taken, And.
The dea requires a physical inventory of all controlled substances to be conducted every. Controlled substances initial/biennial inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location.