Can Ammonia Form Hydrogen Bonds
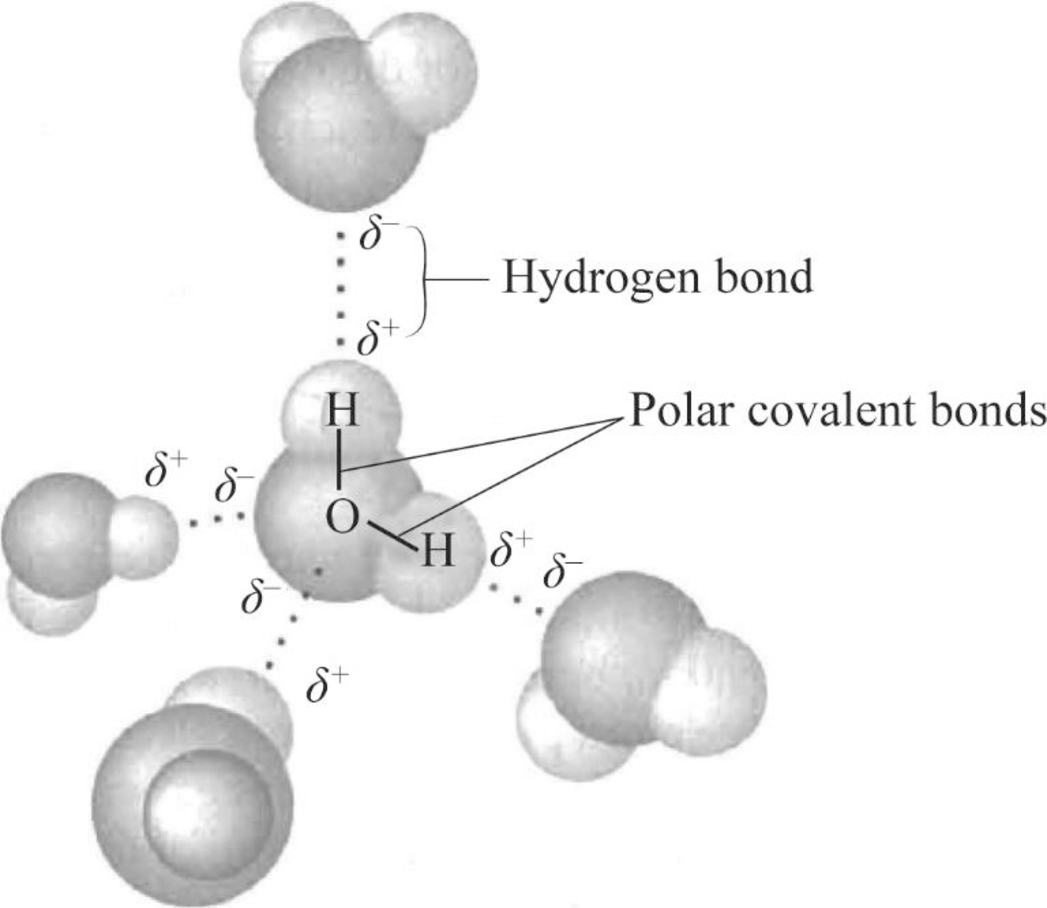
Can Ammonia Form Hydrogen Bonds - Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. The nitrogen atom in ammonia has a lone pair. Yes, ammonia (nh3) can form hydrogen bonds.
Yes, ammonia (nh3) can form hydrogen bonds. Revision notes on hydrogen bonding for the edexcel international as chemistry. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. The nitrogen atom in ammonia has a lone pair.
Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. The nitrogen atom in ammonia has a lone pair. Yes, ammonia (nh3) can form hydrogen bonds. Revision notes on hydrogen bonding for the edexcel international as chemistry.
Where Do Hydrogen Bonds Form
Revision notes on hydrogen bonding for the edexcel international as chemistry. Yes, ammonia (nh3) can form hydrogen bonds. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. The nitrogen atom in ammonia has a lone pair.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas
Yes, ammonia (nh3) can form hydrogen bonds. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. The nitrogen atom in ammonia has a lone pair.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair. Revision notes on hydrogen bonding for the edexcel international as chemistry. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,.
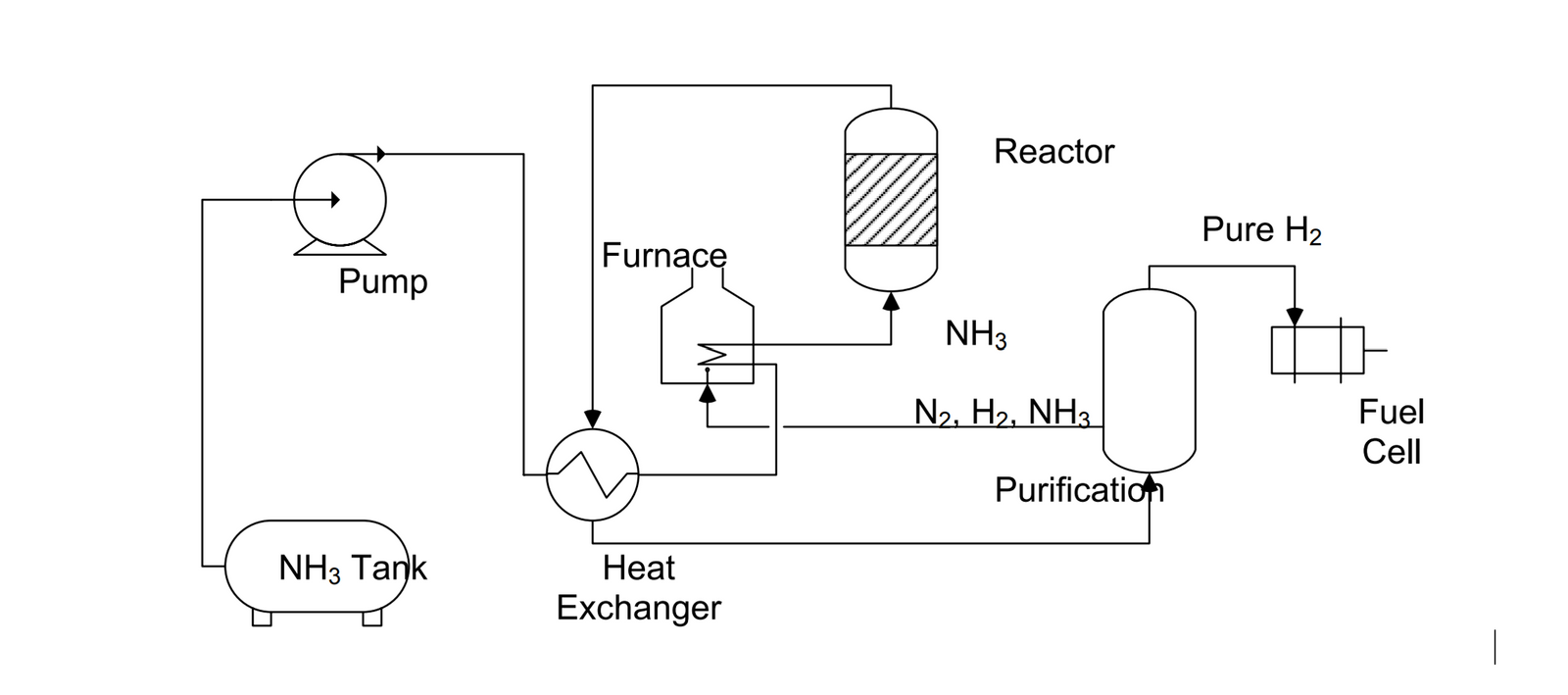
Ammonia as a hydrogen carrier
Yes, ammonia (nh3) can form hydrogen bonds. Revision notes on hydrogen bonding for the edexcel international as chemistry. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. The nitrogen atom in ammonia has a lone pair.
Hydrogen Bonds Chemistry LibreTexts, 55 OFF
Yes, ammonia (nh3) can form hydrogen bonds. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. The nitrogen atom in ammonia has a lone pair.
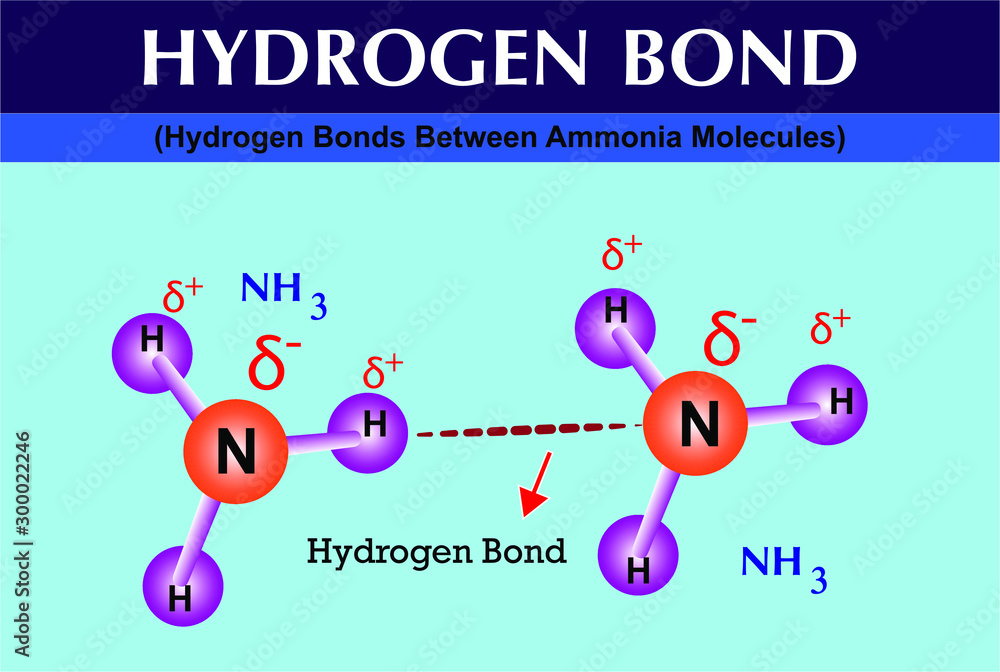
Photo & Art Print Hydrogen Bonds Between Ammonia Molecules, Fridas
Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. The nitrogen atom in ammonia has a lone pair. Yes, ammonia (nh3) can form hydrogen bonds.
Method found for pulling hydrogen from ammonia for use as clean fuel
Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. The nitrogen atom in ammonia has a lone pair. Yes, ammonia (nh3) can form hydrogen bonds.
Ammonia is both a donor and an acceptor of hydrogen in hydro Quizlet
The nitrogen atom in ammonia has a lone pair. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Revision notes on hydrogen bonding for the edexcel international as chemistry. Yes, ammonia (nh3) can form hydrogen bonds.
Hydrogen Bonds Overview Examples Expii Hot Sex Picture
Revision notes on hydrogen bonding for the edexcel international as chemistry. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair.
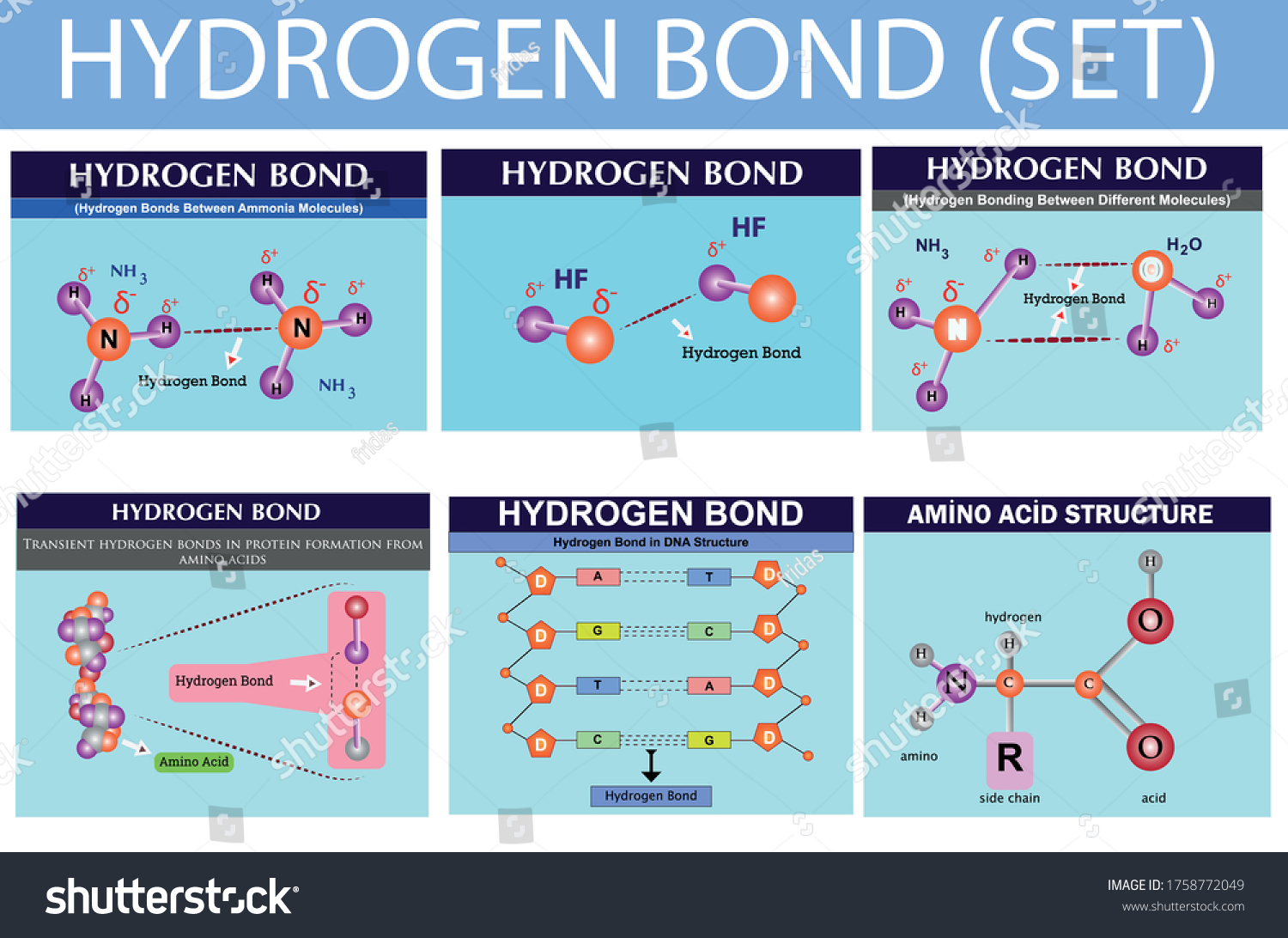
Hydrogen Bond Chemistry Lesson Infographic Hydrogen Stock Vector
The nitrogen atom in ammonia has a lone pair. Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. Yes, ammonia (nh3) can form hydrogen bonds. Revision notes on hydrogen bonding for the edexcel international as chemistry.
Revision Notes On Hydrogen Bonding For The Edexcel International As Chemistry.
Ammonia (nh 3) is intermediate in character between the other two isoelectronic hydrides,. The nitrogen atom in ammonia has a lone pair. Yes, ammonia (nh3) can form hydrogen bonds.